TS Inter 2nd Year – Chemistry Previous Paper 2020
CHEMISTRY, Paper – II
(English Version)
Time: 3 Hours] [Max. Marks: 60
Note: Read the following instructions carefully:
(i) Answer all
(ii) In Section-A, questions from Sr. Nos. 1 to 10 are
(iii) In Section-B, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
(iv)
(v) Draw labelled diagrams wherever necessary for questions in Sections
Section: A
Marks: 10 x 2 = 20
Note: Answer all the questions.
1. State Raoult’s Law.
Answer:
Raoult’s Law states that for an ideal solution, the partial vapor pressure of each component in the solution is directly proportional to its mole fraction.
2. State Faraday’s Law.
Answer:
Faraday’s Laws of Electrolysis describe the relationship between the amount of substance deposited or liberated at an electrode during electrolysis and the quantity of electric charge passed through the electrolyte.
3. Write ores with formulae of the following metals:
(i) Aluminium
Answer:
Bauxite (Al₂O₃·2H₂O)
(ii) Iron
Answer:
Hematite (Fe₂O₃)
4. What is Lanthanoid Contraction?
Answer:
Lanthanoid contraction refers to the steady decrease in the atomic and ionic radii of lanthanide elements (elements from Lanthanum to Lutetium) across the series. This occurs due to the poor shielding effect of the 4f electrons, which results in an increase in the effective nuclear charge experienced by the outer electrons.
5. What are Polymers? Give an example.
Answer:
Polymers are large molecules composed of many repeating smaller units called monomers. They are formed through a process called polymerization.
Example: Polyethylene (made from ethylene monomers)
6. What is vulcanization of rubber?
Answer:
Vulcanization is a process of treating raw rubber with sulfur to improve its properties like elasticity, durability, and resistance to heat and cold. It involves heating rubber with sulfur, which forms cross-links between the polymer chains, making the rubber more rigid and less prone to deformation.
7. What are antacids? Give an example.
Answer:
Antacids are substances that neutralize excess stomach acid (hydrochloric acid). They provide relief from heartburn, indigestion, and acid reflux.
Example: Calcium carbonate (Tums), magnesium hydroxide (Milk of Magnesia)
8. What is tincture of iodine? What is its use?
Answer:
Tincture of iodine is an antiseptic solution containing iodine in alcohol.
Use: It is used to disinfect wounds and prevent infection.
9. What are Enantiomers?
Answer:
Enantiomers are a pair of stereoisomers that are mirror images of each other and cannot be superimposed. They are like left and right hands – they have the same structure but are non-identical.
10. What are ambident nucleophiles?
Answer:
Ambident nucleophiles are nucleophiles that can attack an electrophile from two different sites. They have two nucleophilic centers.
Example:
The nitrite ion (NO₂⁻) can attack through the nitrogen atom or the oxygen atom.
Section: B
Marks: 6 x 4 = 24
Note: Answer any six questions.
11. Derive Bragg’s equation.
Answer:
Bragg’s Law describes the conditions for constructive interference when X-rays are diffracted by the planes of atoms in a crystal lattice.
Derivation:
Consider a beam of X-rays incident on a crystal lattice.
The X-rays are scattered by the atoms in the crystal planes.
For constructive interference to occur, the path difference between the waves scattered from adjacent planes must be an integral multiple of the wavelength (λ).
If the angle of incidence of the X-rays on the crystal plane is θ, the path difference is 2d sin θ, where d is the distance between the planes.
Therefore, for constructive interference:
where n is an integer (1, 2, 3, …).
This is Bragg’s Law, which relates the angle of incidence, the interplanar spacing, and the wavelength of the X-rays.
12. Define molarity. Calculate the molarity of a solution containing 5 g of NaOH in 450 ml solution.
Answer:
Molarity is defined as the number of moles of solute dissolved per liter of solution.
Calculation:
Moles of NaOH:
Molar mass of NaOH = 23 (Na) + 16 (O) + 1 (H) = 40 g/mol
Moles of NaOH =
=
= 0.125 moles
Volume of solution:
450 ml = 0.45 L
Molarity =
=
= 0.278 M
Therefore, the molarity of the NaOH solution is 0.278 M.
13. What are different types of adsorption? Give any four differences between characteristics of these different types.
Answer:
Types of Adsorption:
- Physical Adsorption (Physisorption):
- Weak van der Waals forces are responsible for the attraction between adsorbent and adsorbate.
- Low heat of adsorption.
- Reversible process.
- Multilayer adsorption is possible.
- No specificity for adsorbent.
- Chemical Adsorption (Chemisorption):
- Strong chemical bonds (covalent or ionic) are formed between adsorbent and adsorbate.
- High heat of adsorption.
- Irreversible process.
- Monolayer adsorption.
- Highly specific for adsorbent.
Differences:
| Characteristic | Physisorption | Chemisorption |
|---|---|---|
| Forces involved | van der Waals forces | Chemical bonds (covalent/ionic) |
| Heat of adsorption | Low | High |
| Reversibility | Reversible | Irreversible |
| Adsorption type | Multilayer | Monolayer |
14. Explain the purification of sulphide ore by Froth Floatation method.
Answer:
Froth Floatation is a process used to concentrate sulfide ores.
Principle:
The sulfide ore particles are preferentially wetted by oil (pine oil) due to their hydrophobic nature.
Gangue particles, on the other hand, are hydrophilic and are wetted by water.
Procedure:
- The finely ground ore is mixed with water and a frothing agent (like pine oil).
- Air is blown through the mixture to produce froth.
- The sulfide ore particles attach to the air bubbles and are carried to the surface as froth.
- The froth is skimmed off, and the sulfide ore is recovered.
- The gangue particles settle at the bottom.
15. Explain the terms:
- (a) Ligand:
A ligand is a molecule or ion that can donate a lone pair of electrons to a central metal atom or ion to form a coordinate covalent bond. - (b) Co-ordination number:
The coordination number of a central metal atom or ion is the number of ligands directly attached to it. - (c) Co-ordination entity:
A coordination entity is a central metal atom or ion bonded to a fixed number of ligands. - (d) Central metal atom/ion:
The metal atom or ion that is surrounded by ligands in a coordination compound.
16. How are XeF₂ and XeF₄ prepared? Give their structure.
Answer:
XeF₂ Preparation:
Xenon gas is directly reacted with fluorine gas in a nickel vessel at 400°C.
XeF₄ Preparation:
Xenon gas is reacted with excess fluorine gas at 400°C under high pressure.
Structures:
- XeF₂: Linear (Xe-F-F)
- XeF₄: Square planar
17. What are hormones? Give one example for each:
- (a) Steroid hormone:
Steroid hormones are derived from cholesterol.
Example: Testosterone - (b) Polypeptide hormones:
Polypeptide hormones are chains of amino acids.
Example: Insulin - (c) Amino acid derivatives:
Amino acid derivatives are hormones derived from a single amino acid.
Example: Thyroxine (derived from tyrosine)
18. Write the IUPAC names of the following compounds:
- (a) NH₂-CH₂-CH=CH₂
Answer: 3-Aminoprop-1-ene - (b) C₂H₅-N-CH₂-CH₂-CH₂-CH₃
Answer: N-Ethylbutylamine
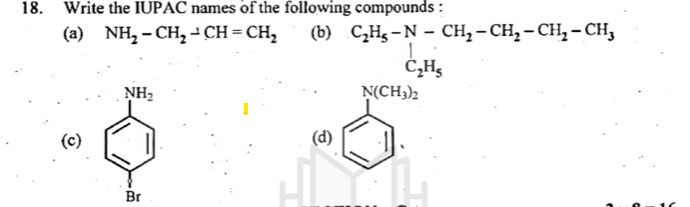
18. Write the IUPAC names of the following compounds:
(a) NH₂-CH₂-CH=CH₂
-
IUPAC Name: 3-Aminoprop-1-ene
-
Explanation:
- The longest carbon chain with the double bond is 3 carbons (propene).
- The amino group (NH₂) is on the third carbon.
(b) C₂H₅-N-CH₂-CH₂-CH₂-CH₃
-
IUPAC Name: N-Ethylbutylamine
-
Explanation:
- The longest carbon chain attached to the nitrogen is 4 carbons (butyl).
- An ethyl group (C₂H₅) is attached to the nitrogen.
**(c) **
-
IUPAC Name: 4-Bromoaniline
-
Explanation:
- The parent structure is benzene with an amino group (-NH₂) at position 1 (assumed).
- A bromine atom (Br) is at position 4.
**(d) **
-
IUPAC Name: N,N-Dimethylaniline
-
Explanation:
- The parent structure is benzene with an amino group (-NH₂) at position 1 (assumed).
- Two methyl groups (-CH₃) are attached to the nitrogen atom.
Section: C
Marks: 2 x 8 = 16
Note: Answer any two questions.
19. (a) What are Galvanic Cells? Explain the working of a galvanic cell with a neat sketch taking the Daniel cell as an example.
Answer:
Galvanic Cells:
Galvanic cells are electrochemical cells that convert chemical energy into electrical energy.
They operate on spontaneous redox reactions. In these cells, oxidation occurs at one electrode (anode), and reduction occurs at the other electrode (cathode).
Working of a Galvanic Cell (Daniel Cell):
Construction:
A Daniel cell consists of two half-cells:
- Anode: A zinc rod dipped in a solution of zinc sulfate (ZnSO₄).
- Cathode: A copper rod dipped in a solution of copper sulfate (CuSO₄).
The two half-cells are connected by a salt bridge (a U-shaped tube filled with a salt solution like KCl).
Process:
-
Anode (Oxidation): Zinc metal oxidizes to zinc ions and releases electrons:
-
Cathode (Reduction): Copper ions in the solution accept electrons and are reduced to copper metal:
-
The electrons flow from the zinc electrode (anode) to the copper electrode (cathode) through the external circuit.
-
The salt bridge maintains electrical neutrality in both half-cells by allowing the flow of ions.
Overall Reaction:
19. (b) What are fuel cells? Give the construction of an H₂-O₂ fuel cell.
Answer:
Fuel Cells:
Fuel cells are electrochemical devices that convert the chemical energy of a fuel (like hydrogen) directly into electrical energy through a continuous electrochemical reaction.
They are similar to batteries, but differ in that the reactants are continuously supplied from an external source.
Construction of H₂-O₂ Fuel Cell:
-
Anode: Hydrogen gas is oxidized at the anode in the presence of a catalyst (like platinum):
-
Cathode: Oxygen gas is reduced at the cathode in the presence of a catalyst:
-
Electrolyte: A proton-exchange membrane (PEM) separates the anode and cathode compartments and allows the passage of protons.
Overall Reaction:
20. (a) How is Ammonia manufactured by the Haber Process?
Answer:
Haber’s Process:
It is an industrial process for the synthesis of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂).
Reaction:
Conditions:
- Temperature: 400-500 °C (high temperature favors the forward reaction, but also favors the reverse reaction).
- Pressure: High pressure (around 200-300 atm) favors the forward reaction, as there are fewer moles of gas on the product side.
- Catalyst: Iron catalyst with small amounts of potassium oxide and aluminum oxide as promoters.
20. (b) How does Ozone react with the following:
-
(i) PbS:
Ozone oxidizes lead sulfide (PbS) to lead sulfate (PbSO₄).
-
(ii) KI:
Ozone oxidizes potassium iodide (KI) to iodine (I₂).
-
(iii) Hg:
Ozone reacts with mercury (Hg) to form mercuric oxide (HgO).
-
(iv) Ag:
Ozone does not react with silver (Ag) under normal conditions.
21. Explain the following reactions with equations:
-
(i) Hell-Volhard-Zelinsky reaction (HVZ):
The HVZ reaction is a method for converting carboxylic acids with α-hydrogens into α-haloacids.
Mechanism: In the presence of phosphorus and chlorine (or bromine), the α-hydrogen of a carboxylic acid is replaced by a halogen atom.
Example:
-
(ii) Decarboxylation:
Decarboxylation is the process of removing a carboxyl group (-COOH) from a carboxylic acid, typically yielding carbon dioxide (CO₂) as a byproduct.
Example:
-
(iii) Aldol Condensation:
Aldol condensation is a reaction in which two molecules of an aldehyde or ketone react to form a β-hydroxy aldehyde or ketone (aldol).
Mechanism: Involves the formation of an enolate ion, which acts as a nucleophile and attacks the carbonyl carbon of another molecule.
Example:
-
(iv) Gatterman-Koch Reaction:
The Gatterman-Koch reaction is a method for converting aromatic compounds to benzaldehydes.
Mechanism: Aryl diazonium salts react with carbon monoxide in the presence of hydrochloric acid.
Example: