2023 – Physical Science
Section -I.
Each question carries 2 marks
1. Mention the applications of the thermite process in our daily life:
The thermite process is a highly exothermic reaction between a metal powder (like aluminum) and metal oxide (like iron oxide) to produce intense heat and a molten metal. The applications of the thermite process include:
- Welding: Used for joining heavy metal parts in railway tracks, pipelines, and large machinery.
- Military Use: Employed in incendiary bombs, as the reaction produces intense heat capable of causing fires.
- Metal Cutting: Used in cutting through thick metal parts, especially in industries and salvage operations.
- Production of Pure Metals: Helps in the extraction of metals like aluminum from metal oxides.
2. A person is viewing an extended object. If a converging lens is placed in front of his eye, will he feel that the size of the object has increased? Why?
Yes, the person will feel that the size of the object has increased. This happens because a converging lens (also known as a convex lens) magnifies objects. When a person views an object through a converging lens, the lens bends the light rays in such a way that the image of the object appears larger. This magnification occurs due to the lens’s ability to focus the diverging rays from the object into a converging point, creating an enlarged image on the retina.
3. List out the materials required to verify Ohm’s law:
To verify Ohm’s Law, the following materials are required:
- Power Supply (Battery or DC power source) – To provide a constant voltage.
- Resistor – To provide a known resistance in the circuit.
- Ammeter – To measure the current flowing through the circuit.
- Voltmeter – To measure the voltage across the resistor.
- Rheostat – A variable resistor to adjust the current.
- Connecting Wires – To connect the components in the circuit.
- Switch – To control the flow of current in the circuit.
By varying the voltage and measuring the current, Ohm’s law (V = IR) can be verified by confirming that the current is directly proportional to the applied voltage for a constant resistance.
SECTION – II
Each question. carries 4 marks
4. Find the distance of the image, and height of the image, when an object height is 4 cm. is placed on the principal axis at a distance of 10 cm in front of a concave mirror whose radius of curvature is 8 cm.
To solve this, we can use the mirror formula and magnification formula:
Given:
- Object distance,
(negative sign because it is in front of the mirror)
- Radius of curvature,
- Focal length,
Mirror Formula:
where,
is the focal length,
is the image distance, and
is the object distance.
Substituting the known values:
Solving for
:
So, the image distance is approximately 2.86 cm, and it is formed in front of the mirror (since
is positive for a concave mirror).
Magnification Formula:
Substituting the known values:
So, the height of the image is:
The negative sign indicates that the image is inverted.
Summary:
- Image Distance: 2.86 cm (real, in front of the mirror)
- Height of Image: -1.144 cm (inverted, smaller than the object)
5. In an atom, the number of electrons in M-shell is equal to the number of electrons in the K-shell and L-shell. Answer the following questions:
Given that the number of electrons in the M-shell is equal to the number of electrons in the K-shell and L-shell, let’s break down the information:
a) Which is the outer-most shell?
- The outer-most shell is the M-shell since it is mentioned that M-shell has the same number of electrons as the K and L shells combined.
b) How many electrons are there in its outer-most shell?
- The total number of electrons in the K-shell (2), L-shell (8), and M-shell (also 8) is 18.
- Therefore, the number of electrons in the M-shell (outer-most shell) is 8 electrons.
c) What is the atomic number of the element?
- The atomic number is the total number of electrons in the neutral atom, which is the sum of electrons in all shells:
So, the atomic number is 18.
d) Write the electronic configuration of the element:
- Since the atomic number is 18, the electronic configuration of the element is:
This is the configuration for Argon (Ar), a noble gas.
6. Write the Allotropes of Carbon and their applications in our daily life:
Allotropes of Carbon:
- Diamond:
- Hardest known natural material.
- Transparent and used in jewelry.
- Used in cutting, grinding, and drilling tools due to its hardness.
- Graphite:
- Soft and slippery.
- Used in pencils as the writing material (commonly called pencil lead).
- Used as a lubricant in machinery.
- Acts as an electrode material in batteries.
- Fullerenes:
- A form of carbon molecule, usually in the shape of a sphere, ellipsoid, or tube (e.g., buckyballs and nanotubes).
- Used in research for nanotechnology, electronics, and medicine.
- Graphene:
- A single layer of carbon atoms arranged in a 2D lattice.
- Extremely strong and conductive, used in electronics, sensors, and energy storage devices.
- Amorphous Carbon:
- Carbon that does not have a well-defined structure.
- Found in charcoal, soot, and coal.
- Used in filtration, black pigments in inks, and as a reducing agent in chemical processes.
Applications in Daily Life:
- Diamond is used in cutting tools and jewelry due to its hardness and brilliance.
- Graphite is used in writing instruments (pencils), batteries, and as a lubricant.
- Fullerenes and Graphene are increasingly used in nanotechnology, energy storage, and electronics.
- Amorphous Carbon is widely used in filtration and as a black pigment in inks.
SECTION – III
Each question carries – 6 marks
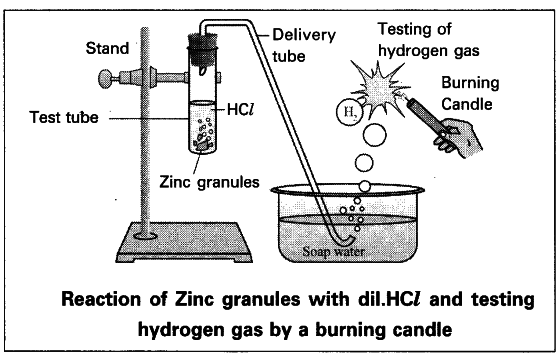
7. Write experimental procedure of a metal reacting with acid. Draw a neat diagram:
Objective: To observe the reaction of a metal (e.g., zinc) with an acid (e.g., hydrochloric acid) and study the products formed.
Materials Required:
- Metal (e.g., zinc strip or zinc granules)
- Dilute hydrochloric acid (HCl)
- Test tube
- Delivery tube
- Water bath or beaker
- Burner (optional for heating)
- Gas jar (to collect gas)
- Glass rod
- Bunsen burner (if heating is required)
Experimental Procedure:
- Setup the Apparatus:
Take a test tube and place a few granules or a small strip of zinc metal inside it. - Add Acid:
Pour dilute hydrochloric acid into the test tube containing the zinc metal. - Observe the Reaction:
As soon as the zinc reacts with hydrochloric acid, bubbles of gas will be produced. This gas is hydrogen gas. The reaction is as follows: - Collect the Gas (Optional):
If you want to collect the hydrogen gas, attach a delivery tube to the test tube and direct the gas into an inverted water-filled jar to collect it. - Confirm the Gas is Hydrogen (Optional):
To confirm the gas produced is hydrogen, bring a burning match near the opening of the jar. If the gas is hydrogen, it will ignite with a characteristic “pop” sound.
Observations:
- The zinc metal reacts with hydrochloric acid, producing hydrogen gas and zinc chloride solution.
- A “pop” sound confirms the presence of hydrogen gas.
Diagram:
8. Write the factors of Ionization energy and how it changes in groups and periods:
Ionization Energy (IE):
Ionization energy is the amount of energy required to remove an electron from an atom or ion in the gas phase.
Factors Affecting Ionization Energy:
- Atomic Size:
The larger the atomic size, the lower the ionization energy. This is because the outermost electron is farther from the nucleus and is less attracted to it. - Nuclear Charge:
The higher the nuclear charge (more protons), the greater the attraction between the nucleus and the electrons, which increases ionization energy. - Shielding Effect:
Inner electrons shield the outer electrons from the full charge of the nucleus. More shielding reduces ionization energy. - Electron Configuration:
Atoms with stable electron configurations (like noble gases) tend to have higher ionization energies because removing an electron would disrupt their stability.
Trend in Groups and Periods:
- Across a Period (Left to Right):
- Ionization energy increases.
As you move across a period, the nuclear charge increases, and electrons are added to the same energy level. This increases the attraction between the nucleus and the electrons, making it harder to remove electrons.
- Ionization energy increases.
- Down a Group:
- Ionization energy decreases.
As you move down a group, the atomic size increases, and the outer electrons are farther from the nucleus. This makes it easier to remove an electron because the attraction between the nucleus and outer electron decreases.
- Ionization energy decreases.
9. Draw ray diagrams for convex lens for the following positions and explain the nature and position of the image:
i) Object is placed at C2 (twice the focal length) of a convex lens:
Ray Diagram:
- Position of Object (O): Placed at
, which is twice the focal length (2F) of the lens.
- Position of Image (I): The image is formed at
, which is at the same distance as the object from the lens.
Characteristics of the Image:
- The image is real.
- The image is inverted.
- The image is of the same size as the object.
- The image is formed at a distance equal to the object from the lens (at
ii) Object is placed between F2 and optic center P of a convex lens:
Ray Diagram:
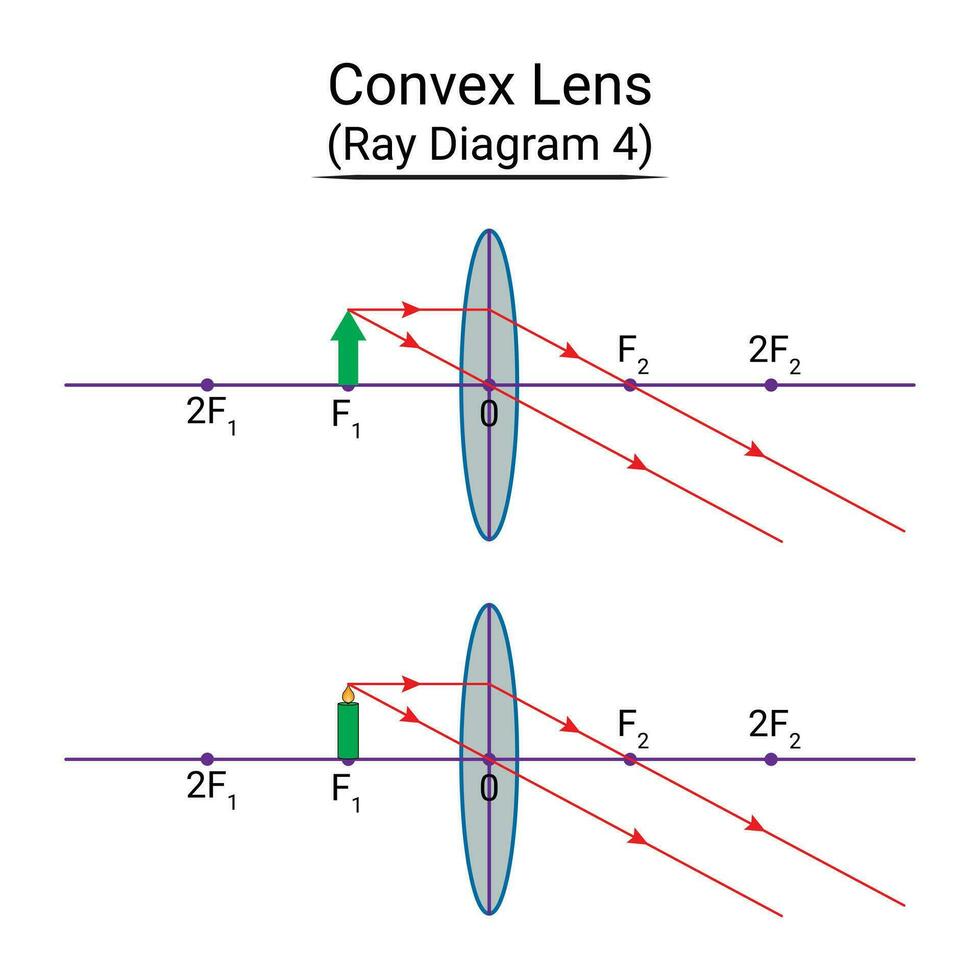
- Position of Object (O): Placed between
(focal point) and the optical center
- Position of Image (I): The image is formed beyond 2F.
Characteristics of the Image:
- The image is real.
- The image is inverted.
- The image is magnified (larger than the object).
- The image is formed on the opposite side of the object, beyond