CBSE Class 12 –Chemistry Question Paper 2022
SECTION-A
1. Define rate of reaction. Write two factors that affect the rate of reaction.
Answer: The rate of reaction is the change in the concentration of reactants or products per unit time. It is a measure of how fast or slow a reaction occurs. Two factors that affect the rate of reaction are:
- Concentration of Reactants: Higher concentration of reactants generally increases the rate of reaction.
- Temperature: An increase in temperature typically speeds up the rate of reaction by providing more energy for collisions between particles.
2. Arrange the following compounds as directed:
(i) In decreasing order of basic strength in aqueous solution:
- C2H5NH2, (CH3)2NH, (C2H2)N
Answer: In decreasing order of basic strength in aqueous solution: (CH3)2NH > C2H5NH2 > (C2H2)N
(ii) In increasing order of solubility in water:
- (C6H5)2NH, C6H5NH2, CH3NH2
Answer: In increasing order of solubility in water: (C6H5)2NH < C6H5NH2 < CH3NH2
(iii) In decreasing order of their pKb values:
- C6H5NH2, C2H5NH2, NH3
Answer: In decreasing order of their pKb values: NH3 > C2H5NH2 > C6H5NH2
3. Write the Nernst equation for the following cell reaction:
Zn(s) + Cu²⁺ (aq) → Zn²⁺ (aq) + Cu(s)
Answer: The Nernst equation for the cell reaction can be written as:
Where:
is the cell potential at non-standard conditions.
is the standard cell potential.
is the number of electrons involved in the reaction (which is 2 for this reaction).
and
are the concentrations of Zn²⁺ and Cu²⁺ ions, respectively.
How will the
be affected when concentration of:
(i) Cu²⁺ ions is increased?
Answer: When the concentration of Cu²⁺ ions is increased, the term
in the Nernst equation decreases. As a result,
becomes more negative, making
increase. Therefore, increasing the concentration of Cu²⁺ ions will increase the cell potential.
(ii) Zn²⁺ ions is increased?
Answer: When the concentration of Zn²⁺ ions is increased, the term
increases. As a result,
becomes more positive, making
decrease. Therefore, increasing the concentration of Zn²⁺ ions will decrease the cell potential.
SECTION-B
4. Following ions of 3d-transition series are given: Ti⁴⁺, V³⁺, Cr³⁺, Mn³⁺
(Atomic number: Ti = 22, V = 23, Cr = 24, Mn = 25) Identify the ion which is:
(i) Most stable in aqueous solution.
Answer: Ti⁴⁺ is the most stable in aqueous solution.
Reason: Ti⁴⁺ has a high charge and a relatively small size, which makes it highly stable in aqueous solution. Its high charge density leads to a stable hydration shell, making it less likely to undergo hydrolysis or reduction.
(ii) A strong oxidizing agent.
Answer: Mn³⁺ is the strongest oxidizing agent.
Reason: Mn³⁺ has a high oxidation state and is unstable in aqueous solution. It has a strong tendency to accept electrons and get reduced to Mn²⁺, making it a strong oxidizing agent.
(iii) Colourless in aqueous solution.
Answer: Ti⁴⁺ is colourless in aqueous solution.
Reason: Ti⁴⁺ has no d-electrons, and the absence of d-d transitions results in it being colourless in solution.
5. (a) (i) On the basis of crystal field theory, write the electronic configuration for d¹ ion if Δₚ > P.
Answer: For a d¹ ion in an octahedral field with Δₚ > P (strong field ligand):
- The five d-orbitals split into two energy levels: eₖ (higher energy) and t₂ₖ (lower energy).
- With Δₚ > P, the electron will occupy the t₂ₖ orbital (lower energy level).
- Electronic configuration: t₂ₖ¹ (1 electron in the lower energy orbital).
(ii) Using valence bond theory, predict the hybridization and magnetic character of [Ni(CN)₄]²⁻ (Atomic number of Ni = 28).
Answer:
- Oxidation state of Ni: In [Ni(CN)₄]²⁻, Ni is in the +2 oxidation state (since CN⁻ is a monodentate ligand and has a -1 charge).
- Electron configuration of Ni²⁺: Ni (atomic number 28) has the configuration [Ar] 3d⁸ 4s². For Ni²⁺, this becomes [Ar] 3d⁸.
- Hybridization: The CN⁻ is a strong field ligand and will cause pairing of the d-electrons in Ni²⁺. The hybridization of Ni²⁺ in [Ni(CN)₄]²⁻ is sp³.
- Magnetic character: Since all the d-electrons are paired, the complex is diamagnetic.
(iii) Write the formula of the following complex using IUPAC norms: Dichloridobis (ethane-1,2-diamine) cobalt(III).
Answer: The IUPAC name indicates:
- The central metal ion is cobalt with a +3 oxidation state (Co³⁺).
- There are two types of ligands:
- Dichlorido (Cl⁻) as two chloride ions.
- Bis(ethane-1,2-diamine), where “bis” indicates two ethane-1,2-diamine ligands.
- The formula is [CoCl₂(en)₂], where “en” stands for ethane-1,2-diamine (C₂H₄NH₂₂).
(b) When a coordination compound NiCl₂.6H₂O is mixed with AgNO₃, 2 moles of AgCl are precipitated per mole of the compound.
(i) Structural formula of the complex.
Answer: The fact that 2 moles of AgCl are precipitated indicates that there are 2 chloride ions present outside the coordination sphere. Hence, the structure is likely [Ni(H₂O)₆]Cl₂.
(ii) Secondary valency of Ni in the complex.
Answer: The secondary valency of Ni is the number of coordinate bonds formed between Ni and the ligands. In [Ni(H₂O)₆]Cl₂, Ni forms 6 coordinate bonds with water molecules. Therefore, the secondary valency of Ni is 6.
(iii) IUPAC name of the complex.
Answer: The IUPAC name of the complex [Ni(H₂O)₆]Cl₂ is Hexaaquanickel(II) chloride.
6. A first-order reaction is 50% complete in 40 minutes. Calculate the time required for the completion of 90% of the reaction.
Answer:
For a first-order reaction, the equation for the time required to reach a certain percentage completion is given by the formula:
Where:
is the time required.
is the rate constant.
is the initial concentration of the reactant.
is the concentration of the reactant at time
.
First, calculate the rate constant
from the data that the reaction is 50% complete in 40 minutes. For a first-order reaction, when 50% of the reaction is complete:
Using the formula:
Substituting the values:
Now, to calculate the time required for 90% completion,
:
So, the time required for 90% completion of the reaction is approximately 134 minutes.
7. (a) Illustrate the following reactions giving suitable examples in each case:
(i) Gabriel phthalimide synthesis.
Answer: The Gabriel phthalimide synthesis is a method to prepare primary amines. It involves the reaction of phthalimide with an alkyl halide in the presence of a strong base (such as KOH) to form an N-alkyl phthalimide intermediate, which is then hydrolyzed to form the primary amine.
Reaction:
Example:
(ii) Carbylamine reaction.
Answer: The carbylamine reaction is the reaction of primary amines with chloroform (CHCl₃) in the presence of a base (such as KOH) to form an isocyanide (carbylamine).
Reaction:
Example:
(iii) Hoffmann bromamide degradation reaction.
Answer: The Hoffmann bromamide degradation reaction involves the conversion of an amide to a primary amine by reaction with bromine in the presence of an alkali.
Reaction:
Example:
7. (b) Write the structures of A, B, and C in the following reactions:
(You have mentioned a set of reactions but did not provide enough context. Could you please provide more details or the reactions to address this question fully?)
8. (a) Account for the following:
(i) Transition elements have higher enthalpies of atomisation.
Answer: Transition elements have high enthalpies of atomization because they have strong metallic bonds due to the presence of unpaired d-electrons. These d-electrons form a strong bond in the metal lattice, requiring more energy to break the bonds and separate the atoms.
(ii) Separation of a mixture of lanthanoid elements is difficult.
Answer: The separation of lanthanoid elements is difficult due to their similar chemical properties, particularly their similar ionic radii and identical +3 oxidation state. This results in very similar behavior in chemical reactions, making it hard to separate them.
(iii) The
value for copper is positive.
Answer: The
value for copper is positive because copper has a high affinity for electrons, making it easily reduced from its
oxidation state to the metallic form. This leads to a positive reduction potential.
8. (b) Define transition elements. Which of the d-block elements may not be regarded as the transition elements? Why do transition metals generally form coloured compounds?
Answer:
Transition elements are the elements found in the d-block of the periodic table, which have partially filled d-orbitals in at least one of their oxidation states.
d-block elements that may not be regarded as transition elements:
- Zinc (Zn), Cadmium (Cd), and Mercury (Hg) are not considered transition metals because they have fully filled d-orbitals in their most common oxidation states (+2), i.e., their d-orbitals are completely filled and do not participate in bonding.
Why transition metals form coloured compounds:
- Transition metals form coloured compounds because of the presence of partially filled d-orbitals. When light is absorbed, electrons in these d-orbitals undergo d-d transitions, which leads to the absorption of specific wavelengths of light, thus producing visible colour.
9. An organic compound X with the molecular formula C₁₀H₁₀O forms a 2,4-DNP derivative, does not reduce Tollens’ reagent but gives a positive iodoform test on heating with I₂ in the presence of NaOH.
Answer:
(i) Structure of compound X:
Given the molecular formula (C₁₀H₁₀O), the positive iodoform test indicates the presence of a methyl ketone group (-COCH₃). The reaction with 2,4-DNP suggests the presence of a carbonyl group, indicating that the compound is likely a benzaldehyde or acetophenone derivative. The fact that it does not reduce Tollens’ reagent indicates it is not an aldehyde, so the compound is likely acetophenone (C₆H₅COCH₃).
(ii) Structure of the product obtained when compound X reacts with 2,4-DNP reagent:
When acetophenone reacts with 2,4-DNP, a yellow or orange precipitate of the derivative is formed. The product is a 2,4-DNP derivative of acetophenone (a hydrazone).
(iii) Structures of the products obtained when compound X is heated with I₂ in the presence of NaOH:
The positive iodoform test indicates that acetophenone reacts with iodine and sodium hydroxide to form iodoform (CHI₃). The reaction produces:
- Acetophenone is oxidized to form acetic acid (CH₃COOH).
- Iodoform (CHI₃) is formed as a result of the reaction.
10. (a) Write any three differences between physisorption and chemisorption.
Answer:
- Physisorption: Involves weak Van der Waals forces, whereas chemisorption involves strong covalent or ionic bonds.
- Physisorption: It is a reversible process, while chemisorption is generally irreversible.
- Physisorption: Occurs at low temperatures and does not require activation energy, whereas chemisorption occurs at higher temperatures and requires activation energy.
SECTION-C
12. Read the passage given below and answer the questions that follow:
Aldehydes, ketones and carboxylic acids are some of the important classes of organic compounds containing carbonyl group. These are highly polar molecules due to higher electro-negativity of oxygen relative to carbon in the carbonyl group. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes.
Aldehydes and ketones undergo nucleophilic addition reactions onto the carbonyl group but carboxylic acid does not undergo nucleophilic addition reaction. The alpha (a) – hydrogens of aldehydes and ketones are acidic. Therefore aldehydes and ketones having at least one a-hydrogen undergo Aldol condensation.
Aldehydes are easily oxidised by mild oxidising agents such as Tollens’ reagent and Fehling’s reagent. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and by hydrolysis of nitriles. Aromatic carboxylic acids are prepared by side-chain oxidation of alkyl benzenes. Carboxylic acids are considerably more acidic than alcohols and most of simple phenols.
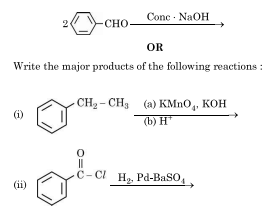
(a) Arrange the following in the increasing order of their reactivity towards nucleophilic addition reaction.:
CH COCH, CH CHO, HCHO, C,H,COCH
(b) Give a simple chemical test to distinguish between Ethanal and Propanone.
(c) Why carboxylic acid does not give nucleophilic addition reactions like aldehydes and ketones?
(d) (i) Why a-hydrogen of aldehydes and ketones are acidic in nature?
(ii) Write the products in the following:
Answers:
a) The increasing order of reactivity towards nucleophilic addition is:
C₆H₅COCH₃ < CH₃COCH₃ < CH₃CHO < HCHO
(b) To distinguish between Ethanal (CH₃CHO) and Propanone (CH₃COCH₃), you can use Tollens’ reagent.
- Ethanal will react with Tollens’ reagent (ammoniacal silver nitrate) to form a silver mirror, indicating the presence of an aldehyde.
- Propanone will not react with Tollens’ reagent, as it is a ketone.
(c) Carboxylic acids do not readily undergo nucleophilic addition reactions like aldehydes and ketones due to the presence of the carboxyl group (-COOH). The carbonyl carbon in carboxylic acids is less electrophilic compared to aldehydes and ketones due to resonance involving the lone pair of electrons on the oxygen atom of the hydroxyl group (-OH). This resonance reduces the positive charge density on the carbonyl carbon, making it less susceptible to nucleophilic attack. Additionally, the negative charge on the carboxylate anion (formed after nucleophilic addition) is delocalized, making the addition less favorable.
(d) (i) The α-hydrogen atoms of aldehydes and ketones are acidic due to the strong electron-withdrawing nature of the carbonyl group (C=O). This withdrawal of electrons stabilizes the conjugate base (carbanion) formed after the removal of a proton (H⁺), making the hydrogen more acidic. Also, the carbanion is stabilized by resonance.
(ii) The reaction you provided is incomplete. It shows a reactant (2-CHO- or something similar) reacting with concentrated NaOH, which suggests a possible Cannizzaro reaction or Aldol condensation. Please provide the full reaction or specific reactants for a complete answer.
Assuming it’s a Cannizzaro reaction with two moles of an aldehyde without an α-hydrogen (like formaldehyde or benzaldehyde):
If the reactant is 2 moles of HCHO (formaldehyde), the products would be:
CH₃OH (methanol) + HCOONa (sodium formate)
If the reactant is 2 moles of C₆H₅CHO (benzaldehyde), the products would be:
C₆H₅CH₂OH (benzyl alcohol) + C₆H₅COONa (sodium benzoate)