CBSE Class 10 – Science Previous Paper 2023
Subject : Science
Time allowed : 3 hours
Marks : 80
General Instructions:
- Total Questions: 39, all compulsory.
- Sections:
- Section A (MCQs): Q1-20, 1 mark each.
- Section B (VSA): Q21-26, 2 marks each (30-50 words).
- Section C (SA): Q27-33, 3 marks each (50-80 words).
- Section D (LA): Q34-36, 5 marks each (80-120 words).
- Section E (Case-based): Q37-39, 4 marks each (with sub-parts).
- No overall choice, but internal choice in some sections.
- Follow the word limits for each type of question.
Question:
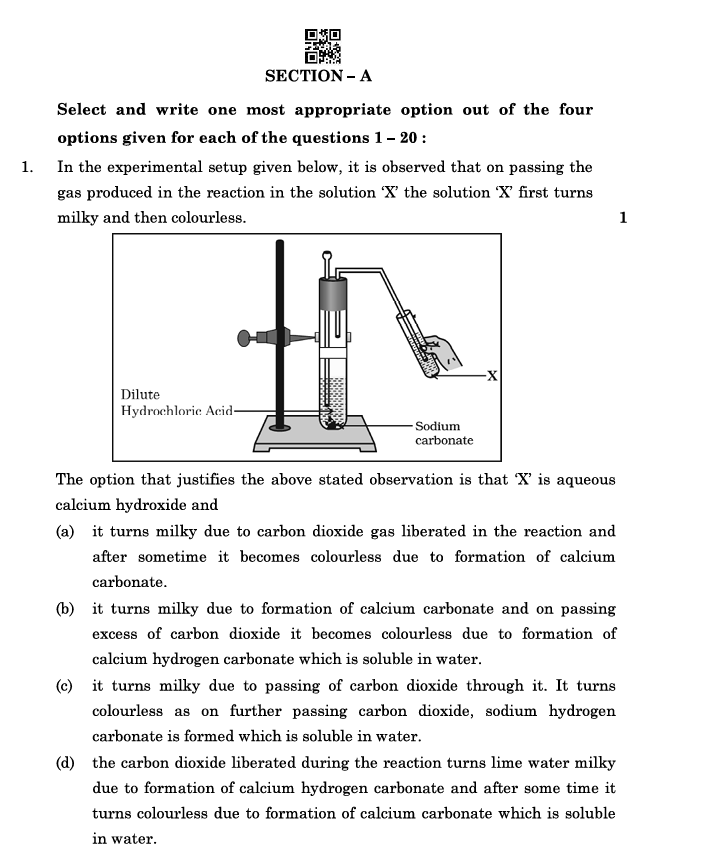
In the experimental setup given below, it is observed that on passing the gas produced in the reaction in the solution ‘X,’ the solution ‘X’ first turns milky and then colourless.
The option that justifies the above-stated observation is that ‘X’ is aqueous calcium hydroxide and:
(a) It turns milky due to carbon dioxide gas liberated in the reaction, and after some time, it becomes colourless due to the formation of calcium carbonate.
(b) It turns milky due to the formation of calcium carbonate, and on passing excess carbon dioxide, it becomes colourless due to the formation of calcium hydrogen carbonate, which is soluble in water.
(c) It turns milky due to passing carbon dioxide through it. It turns colourless as, on further passing carbon dioxide, sodium hydrogen carbonate is formed, which is soluble in water.
(d) The carbon dioxide liberated during the reaction turns lime water milky due to the formation of calcium hydrogen carbonate, and after some time, it turns colourless due to the formation of calcium carbonate, which is soluble in water.
Answer:
(b) It turns milky due to the formation of calcium carbonate, and on passing excess carbon dioxide, it becomes colourless due to the formation of calcium hydrogen carbonate, which is soluble in water.
Explanation:
- Lime water (aqueous calcium hydroxide) turns milky when carbon dioxide is passed through it due to the formation of insoluble calcium carbonate (CaCO₃).
- On passing excess carbon dioxide, the calcium carbonate dissolves to form calcium hydrogen carbonate [Ca(HCO₃)₂], which is soluble in water, turning the solution clear again.
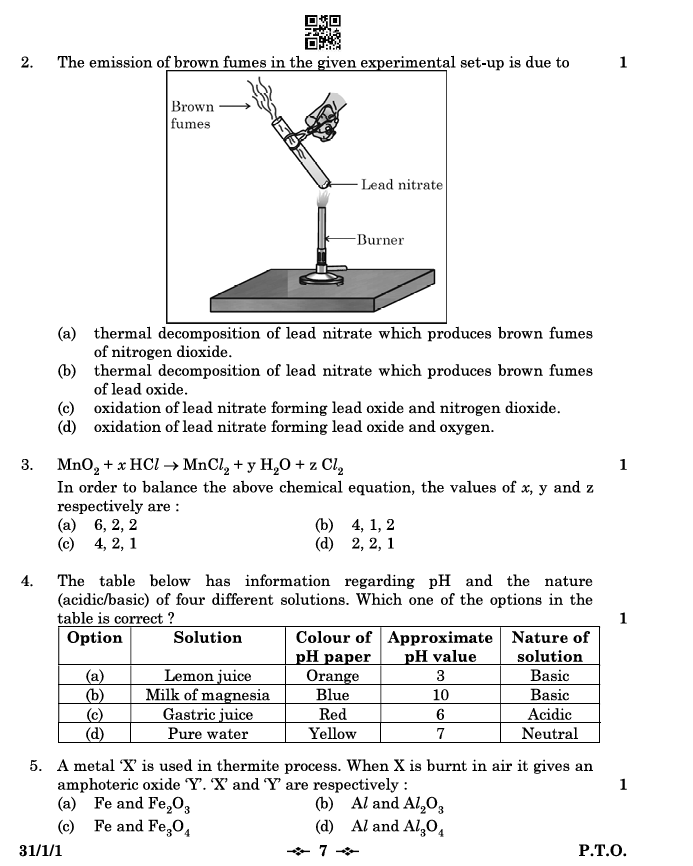
2. The emission of brown fumes in the given experimental setup is due to:
- Answer: (a) Thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
Explanation: Lead nitrate (Pb(NO₃)₂) decomposes on heating to produce lead oxide (PbO), nitrogen dioxide (NO₂) – the brown fumes – and oxygen (O₂).
Reaction:
3. To balance the equation:
Answer: (b) 4, 1, 2
Balanced equation:
4. Identify the correct row in the table about pH:
- Answer: (c) Gastric juice – Red – pH 6 – Acidic
Explanation: - Lemon juice (pH ~2-3) is acidic, not basic.
- Milk of magnesia is basic with a blue pH paper.
- Gastric juice is acidic with a low pH.
- Pure water has a neutral pH of 7.
5. The metal ‘X’ used in the thermite process that gives an amphoteric oxide:
- Answer: (d) Al and Al₂O₃
Explanation:
Aluminum (Al) is used in the thermite process, and aluminum oxide (Al₂O₃) is amphoteric (reacts with acids and bases).

6. Select washing soda from the following:
- Answer: (c) Na₂CO₃·10H₂O
Explanation: - Washing soda is sodium carbonate decahydrate (Na₂CO₃·10H₂O).
- NaHCO₃ is baking soda.
- Na₂CO₃·5H₂O is not a commonly used form of washing soda.
- NaOH is caustic soda.
7. Identify the correct option for the given cyclic carbon compounds:
- Answer: (c) A is a saturated cyclic hydrocarbon, and B and C are unsaturated cyclic hydrocarbons.
Explanation: - A is cyclohexane (C₆H₁₂), a saturated cyclic hydrocarbon (all single bonds).
- B and C are benzene (C₆H₆) and its resonance structure, both unsaturated hydrocarbons due to alternating double bonds.
8. An organism that breaks down food material outside the body and absorbs it:
- Answer: (d) a fungi, Rhizopus
Explanation: - Fungi, like Rhizopus, break down food externally using enzymes and then absorb nutrients (saprophytic nutrition).
- Cuscuta is a plant parasite.
- Tapeworm is an animal parasite.
- Rhizobium is a nitrogen-fixing bacteria, not a decomposer.
9. Consider the following statements about the small intestine and select the one which is NOT correct:
- (a) The length of the small intestine in animals differs as it depends on the type of food they eat.
- (b) The small intestine is the site of complete digestion of food.
- (c) The small intestine receives secretions from liver and pancreas.
- (d) The villi of the small intestine absorb water from the unabsorbed food before it gets removed from the body via the anus.
Answer: (d)
Explanation:
The villi absorb nutrients, not water. Water absorption occurs in the large intestine.
10. The statement that correctly describes the characteristic(s) of a gene is:
- (a) In individuals of a given species, a specific gene is located on a particular chromosome.
- (b) A gene is not the information source for making proteins in the cell.
- (c) Each chromosome has only one gene located all along its length.
- (d) All the inherited traits in human beings are not controlled by genes.
Answer: (a)
Explanation:
Genes are located on specific chromosomes and act as the information source for protein synthesis.
11. Select from the following the correct statement about tropic movement in plants:
- (a) It is due to stimulus of touch and temperature.
- (b) It does not depend upon the direction of stimulus received.
- (c) It is observed only in roots and not in stems.
- (d) It is a growth-related movement.
Answer: (d)
Explanation:
Tropic movement is growth-related and occurs in response to a directional stimulus (like light, gravity, or water).
12. Select the INCORRECT match (between the plant and its vegetative part) from the following:
- (a) Bryophyllum, leaf
- (b) Potato, stem
- (c) Money-plant, stem
- (d) Rose, root
Answer: (d)
Explanation:
In a rose plant, stems (not roots) are used for vegetative propagation.
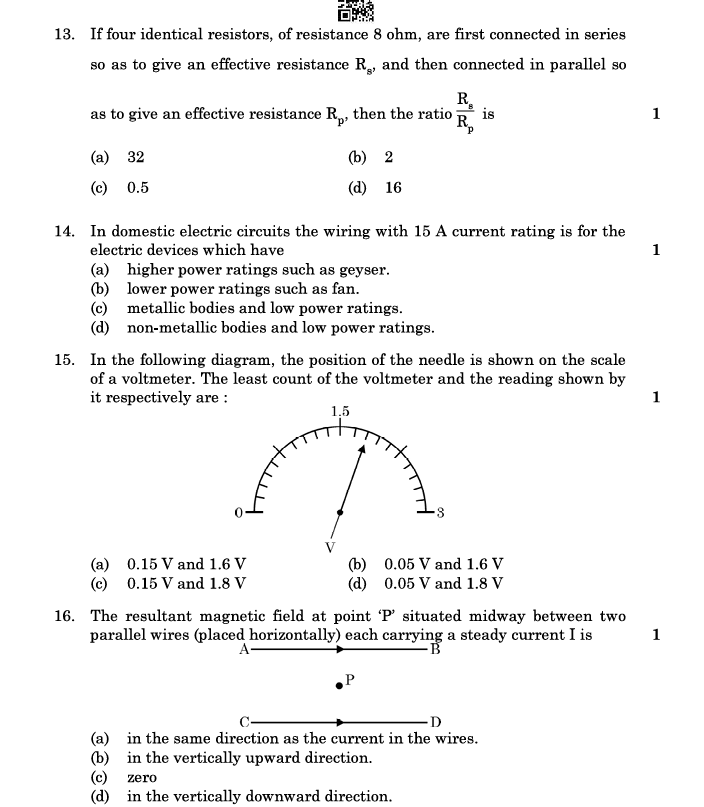
13. If four identical resistors, of resistance 8 ohm, are first connected in series so as to give an effective resistance
, and then connected in parallel so as to give an effective resistance
, then the ratio
is:
- (a) 32
- (b) 2
- (c) 0.5
- (d) 16
Answer: (a) 32
Explanation:
- Series:
- Parallel:
- Ratio:
Wait — let’s recheck the options: - Correct ratio is 16, so the correct answer is (d) 16.
14. In domestic electric circuits, the wiring with 15 A current rating is for the electric devices which have:
- (a) higher power ratings such as geyser.
- (b) lower power ratings such as fan.
- (c) metallic bodies and low power ratings.
- (d) non-metallic bodies and low power ratings.
Answer: (a) higher power ratings such as geyser
Explanation:
Devices like geysers, ACs, and heaters require high current ratings.
15. In the following diagram, the position of the needle is shown on the scale of a voltmeter. The least count of the voltmeter and the reading shown by it respectively are:
- (a) 0.15 V and 1.6 V
- (b) 0.05 V and 1.6 V
- (c) 0.15 V and 1.8 V
- (d) 0.05 V and 1.8 V
Answer: (b) 0.05 V and 1.6 V
Explanation:
- Least count:
- The needle points at 1.6 V.
16. The resultant magnetic field at point ‘P’ situated midway between two parallel wires (placed horizontally) each carrying a steady current
is:
- (a) in the same direction as the current in the wires.
- (b) in the vertically upward direction.
- (c) zero.
- (d) in the vertically downward direction.
Answer: (c) zero
Explanation:
- Equal and opposite currents produce magnetic fields that cancel out midway.
Assertion–Reason Based Questions (Q. Nos. 17 to 20)
Instructions:
Each question consists of two statements—Assertion (A) and Reason (R). Choose the correct option:
- (a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
- (b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
- (c) (A) is true, but (R) is false.
- (d) (A) is false, but (R) is true.
17.
Assertion (A): The colour of aqueous solution of copper sulphate turns colourless when a piece of lead is added to it.
Reason (R): Lead is more reactive than copper and hence displaces copper from its salt solution.
Answer: (c) (A) is true, but (R) is false.
Explanation:
- Lead is indeed more reactive than copper and displaces it, forming lead sulphate (white, not colourless).
- The solution does not turn colourless; it becomes white and cloudy.
18.
Assertion (A): Genes inherited from the parents decide the sex of a child.
Reason (R): X chromosome in a male child is inherited from his father.
Answer: (c) (A) is true, but (R) is false.
Explanation:
- The sex of a child is determined by genes from both parents, specifically whether the father contributes an X or Y chromosome.
- A male inherits his X chromosome from his mother, not his father.
19.
Assertion (A): Blood clotting prevents excessive loss of blood.
Reason (R): Blood clotting is due to blood plasma and white blood cells present in the blood.
Answer: (b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
Explanation:
- Blood clotting prevents excessive blood loss — that’s true.
- However, clotting occurs due to platelets and plasma proteins (not white blood cells).
20.
Assertion (A): The strength of the magnetic field produced at the centre of a current-carrying circular coil increases on increasing the number of turns in it.
Reason (R): The current in each circular turn has the same direction, and the magnetic field due to each turn thus adds up.
Answer: (a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
Explanation:
- Magnetic field strength is directly proportional to the number of turns.
- Since the magnetic fields add up, increasing the turns strengthens the field.
SECTION B: Very Short Answer Questions
Q. 21
(a)
- (i) A compound ‘X’ prepared from gypsum hardens when mixed with water. Identify ‘X’ and write its chemical formula.
- (ii) State the difference in chemical composition between baking soda and baking powder.
OR
(b) Write balanced chemical equations for the reactions when:
- Blue copper sulfate crystals are heated.
- Sodium hydrogen carbonate is heated during cooking.
Answer:
- (i) ‘X’ is Plaster of Paris (POP) – CaSO₄·½H₂O. It forms gypsum when mixed with water.
- (ii)
- Baking soda: Sodium bicarbonate – NaHCO₃
- Baking powder: NaHCO₃ + an edible acid (e.g., tartaric acid).
OR
- (i) CuSO₄·5H₂O → CuSO₄ + 5H₂O (Blue → White)
- (ii) 2NaHCO₃ → Na₂CO₃ + CO₂ + H₂O (Gas released during baking causes dough to rise)
Diagram Required: A simple diagram showing the POP hardening process can be helpful.
Q. 22
(a) Write the role of insulin in regulating blood sugar levels and mention the disease caused by its deficiency.
(b) How is the timing and amount of insulin release regulated?
Answer:
-
(a) Insulin is secreted by the pancreas to regulate blood glucose levels. It helps cells absorb glucose for energy or storage.
- Disease: Diabetes mellitus occurs when insulin production is impaired.
-
(b) Insulin release is regulated by blood glucose levels:
- High blood sugar → More insulin released.
- Low blood sugar → Less insulin secreted.
No diagram required.
Q. 23
(a) Name the type of blood (oxygenated/deoxygenated) transported by:
- Vena cava
- Pulmonary artery
Mention the path of transport in each case.
OR
(b) Draw a schematic flowchart showing the breakdown of glucose for energy:
- In the presence of oxygen.
- In the absence of oxygen.
Answer:
- (a)
- Vena cava: Deoxygenated blood – from the body to the right atrium.
- Pulmonary artery: Deoxygenated blood – from the right ventricle to the lungs for oxygenation.
OR
- (b) Glucose Breakdown Flowchart: (Diagram required)
Presence of Oxygen (Aerobic Respiration)
Glucose → Pyruvate → CO₂ + H₂O + Energy (ATP)
Absence of Oxygen (Anaerobic Respiration)
Glucose → Pyruvate → Lactic Acid (in animals)/Ethanol + CO₂ (in yeast) + Minimal Energy
Diagram Required: A flowchart to show both aerobic and anaerobic respiration pathways.
SECTION B: Very Short Answer Questions (Contd.)
Q. 24
Name the part of the human excretory system where nephrons are found.
Write the structure and function of nephrons. (2 marks)
Answer:
- Nephrons are found in the kidneys.
- Structure: A nephron consists of:
- Bowman’s capsule (glomerulus),
- Proximal tubule, Loop of Henle, Distal tubule, and Collecting duct.
- Function:
- Filtration of blood,
- Reabsorption of essential nutrients,
- Excretion of waste (urine).
Diagram Required: A labeled diagram of a nephron is essential for better understanding.
Q. 25
(a) A narrow beam XY of white light passes through a glass prism ABC (see diagram).
- Trace the path of the emergent beam on a screen PQ.
- Name the phenomenon observed and state its cause. (2 marks)
OR
(b) Age-related vision problems affect the ability to see near and far objects.
- Give the reason for this condition.
- Name the defect that occurs.
- Draw a labeled ray diagram of the corrective lens used to fix it.
Answer:
- (a) Phenomenon: Dispersion of light.
- Cause: The different wavelengths of light refract at different angles when passing through the prism, producing a spectrum of colors.
Diagram Required: Show the incident ray, prism with refracted rays, and the emergent spectrum on screen PQ.
- (b)
- Reason: The lens loses elasticity with age, reducing its accommodating power.
- Defect: Presbyopia (both near and far vision become blurry).
- Corrective Lens: Bifocal lenses – convex for near vision and concave for distant vision.
Diagram Required: A bifocal lens diagram with light rays focusing correctly on the retina.
Q. 26
How do harmful chemicals accumulate progressively at each trophic level in a food chain? (2 marks)
Answer:
- Bio-magnification is the process where toxic substances (e.g., pesticides like DDT) accumulate in organisms higher up the food chain.
- Toxins get stored in tissues and become more concentrated at successive trophic levels.
- Top-level consumers (e.g., birds of prey or humans) often experience the highest toxicity.
No diagram required, but a simple food chain diagram can enhance understanding.
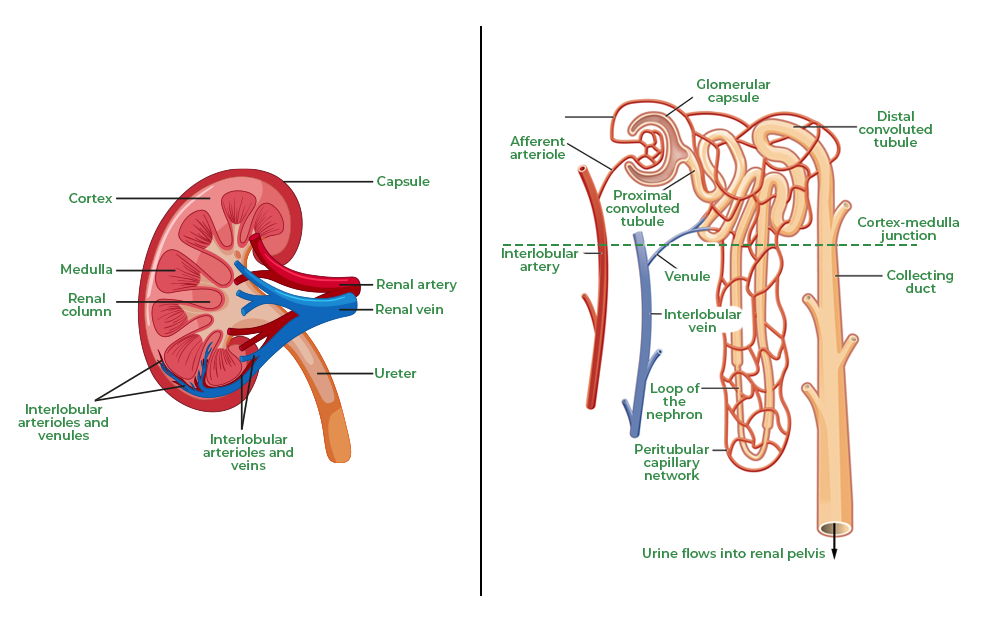
A labeled diagram of a nephron is essential for better understanding.
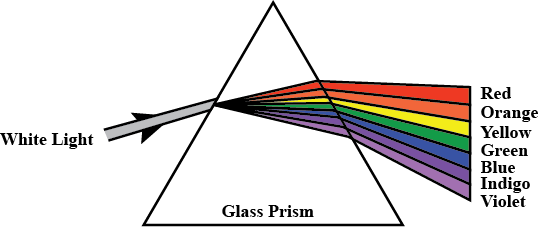
Show the incident ray, prism with refracted rays, and the emergent spectrum on screen PQ.
Question 24:
Name the part of the human excretory system where nephrons are found. Write the structure and function of nephrons.
-
Part: Nephrons are found in the kidneys, specifically in the renal cortex and renal medulla.
-
Structure:
- Glomerulus: A network of capillaries involved in filtration.
- Bowman’s capsule: A cup-like structure surrounding the glomerulus.
- Proximal convoluted tubule (PCT): Reabsorbs nutrients like glucose and amino acids.
- Loop of Henle: Maintains water and salt balance.
- Distal convoluted tubule (DCT): Regulates ion and pH balance.
- Collecting duct: Collects urine to pass into the ureter.
-
Function:
- Filtration of blood to remove waste (urea, creatinine).
- Reabsorption of water, salts, and nutrients.
- Excretion of urine to maintain homeostasis.
Question 25(a):
A narrow beam of white light passes through a glass prism. Trace the path and name the phenomenon.
-
Path of Light:
When white light passes through the prism, it disperses into its constituent colors. The light will refract twice: once upon entering and once upon exiting the prism. On the screen PQ, a spectrum of colors (VIBGYOR) will be visible, with violet deviating the most and red the least. -
Phenomenon: Dispersion of Light.
-
Cause: Different wavelengths of light travel at different speeds through the glass, resulting in varying degrees of bending.
Question 25(b): (Alternative)
Eye power decreases with age.
-
(i) Reason:
With age, the ciliary muscles weaken, and the lens loses elasticity, making it harder to focus on nearby and distant objects. -
(ii) Defect: Presbyopia.
- It affects both near and distant vision due to reduced flexibility of the eye lens.
-
(iii) Corrective Measure:
Bifocal lenses are used, with the upper part for distant vision and the lower part for near vision.
Ray Diagram:
- Show a parallel beam of light focusing correctly on the retina with the help of a bifocal lens.
Question 26:
How do harmful chemicals accumulate at each trophic level in a food chain?
- Process: Biomagnification (or Bioaccumulation).
- Explanation:
- Harmful chemicals like pesticides (DDT) and heavy metals (mercury) enter the food chain from the environment (e.g., through contaminated water).
- These toxins accumulate in the tissues of organisms.
- As we move up trophic levels (from producers to apex predators), the concentration of these chemicals increases because they are not easily broken down.
Question 27: Redox Reactions
(a) Identify the reducing agent in the following reactions:
-
4NH₃ + 5O₂ → 4NO + 6H₂O
- Reducing agent: NH₃ (Ammonia) (It donates electrons by losing hydrogen and gets oxidized to NO.)
-
H₂O + F₂ → HF + HOF
- Reducing agent: H₂O (Water) (Water loses electrons when oxygen forms a bond with fluorine.)
-
Fe₂O₃ + 3CO → 2Fe + 3CO₂
- Reducing agent: CO (Carbon monoxide) (It donates electrons, gets oxidized to CO₂.)
-
2H₂ + O₂ → 2H₂O
- Reducing agent: H₂ (Hydrogen) (It loses electrons to oxygen and forms water.)
(b) Define a redox reaction:
A redox reaction involves both oxidation (loss of electrons or gain of oxygen) and reduction (gain of electrons or loss of oxygen) occurring simultaneously.
Question 28: pH Changes
(a) Remedial measures for pH changes:
-
Indigestion (Excess acid in the stomach):
- Use of an antacid like magnesium hydroxide (Milk of Magnesia) to neutralize excess acid.
-
Bee/Nettle sting (acidic sting):
- Apply a mild base like baking soda (sodium bicarbonate) to neutralize the acidic venom.
(b) Fresh milk (pH 6) turning into curd:
- The pH decreases (becomes more acidic) due to lactic acid fermentation by bacteria, lowering it below 6.
Question 29: Plant Physiology
(a) Cellular processes:
-
Role of ATP:
- ATP (Adenosine Triphosphate) is the energy currency of the cell, providing energy for metabolic activities like muscle contraction, active transport, and biosynthesis.
-
Gas exchange in plants:
- Stomata, lenticels, and root hairs ensure sufficient gas exchange.
-
Conditions affecting gas diffusion:
- Concentration gradient, temperature, and permeability of the membrane.
(b) Alternative option:
-
Internal energy reserve:
- Plants: Starch
- Animals: Glycogen
-
Desert plant adaptation:
- Desert plants use CAM (Crassulacean Acid Metabolism) photosynthesis, keeping stomata closed during the day to reduce water loss and opening them at night to absorb CO₂.
Question 30: Ray Diagram for Image Formation
(a) Complete the ray diagram:
- Draw rays from the object through the lens or mirror, converging to form the image based on focal points and principal axis.
(b) Nature, position, and size of the image:
- Nature: Real and inverted
- Position: Between focus and center of curvature
- Size: Smaller than the object (diminished)
(c) Sign of image distance (Cartesian sign convention):
- Negative (for real images formed by concave mirrors) since the image forms on the same side as the object.
Question 31: Reasoning Questions
(a) Why are danger signals red?
- Red color is used in danger signals because it has the longest wavelength and undergoes least scattering in the atmosphere, making it visible from a distance.
(b) Why does the sky appear dark at high altitudes?
- At high altitudes, the atmosphere is thinner with fewer air molecules to scatter sunlight. As a result, less Rayleigh scattering occurs, making the sky appear dark.
(c) Why is the path of light visible in a colloidal solution?
- The path of light is visible due to the Tyndall effect, where light is scattered by the colloidal particles present in the solution.
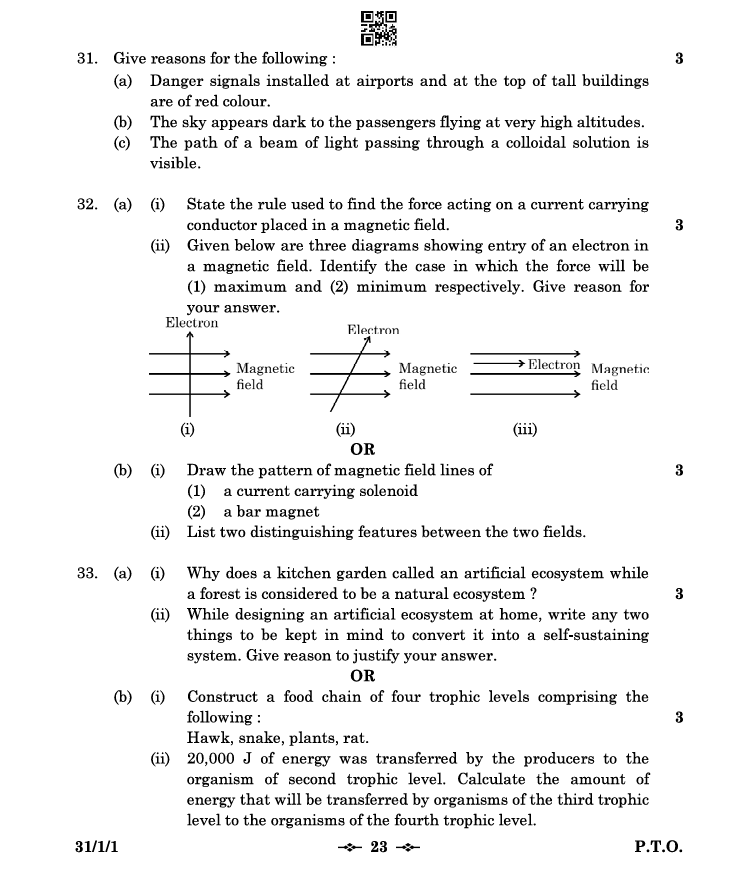
Question 32: Magnetic Fields
(a) Magnetic force on electrons
-
Rule used:
- The Fleming’s Left-Hand Rule states that when the thumb, forefinger, and middle finger of the left hand are stretched mutually perpendicular:
- Thumb: Direction of force
- Forefinger: Direction of magnetic field
- Middle finger: Direction of current
- The Fleming’s Left-Hand Rule states that when the thumb, forefinger, and middle finger of the left hand are stretched mutually perpendicular:
-
Maximum and minimum force:
- Maximum force occurs when the electron moves perpendicular to the magnetic field (diagram (ii)).
- Minimum force occurs when the electron moves parallel or antiparallel to the magnetic field (diagram (iii)).
(b) Magnetic field patterns:
-
Current-carrying solenoid:
- The magnetic field lines resemble those of a bar magnet, with lines emerging from one end (north pole) and entering the other (south pole).
-
Bar magnet:
- Magnetic field lines are continuous loops from the north pole to the south pole outside the magnet and from south to north inside the magnet.
Distinguishing features:
- Solenoid: Field strength can be adjusted by changing current.
- Bar magnet: Field strength is fixed.
Question 33: Ecosystems and Food Chains
(a) Ecosystem types:
-
Kitchen garden – artificial ecosystem:
- It is created and maintained by humans with external inputs like fertilizers, water, and pesticides.
-
Forest – natural ecosystem:
- It is self-sustaining, with natural interactions between biotic and abiotic components.
Steps for self-sustaining artificial ecosystem:
- Maintain diversity of species (producers, consumers, decomposers).
- Ensure nutrient cycling by adding compost and avoiding chemical overuse.
(b) Food chain construction:
Food chain:
Plants → Rat → Snake → Hawk
Energy transfer calculation:
- Energy transferred from second trophic level to third trophic level is governed by the 10% rule:
- Producers → Primary consumers → Secondary consumers → Tertiary consumers
Given: 20,000 J transferred to the second trophic level.
- Energy to third level: 10% of 20,000 J = 2,000 J
- Energy to fourth level: 10% of 2,000 J = 200 J
Answer: 200 J of energy will be transferred from the third trophic level to the fourth trophic level.
SECTION – D
Question 34
(a) Organic Chemistry Process
(i) Identify A, B, and C.
- A: Ethanol (C₂H₆O) – Alcohol series
- B: Ethene (C₂H₄) – Unsaturated hydrocarbon formed by dehydration of ethanol
- C: Ethane (C₂H₆) – Saturated hydrocarbon formed by hydrogenation of ethene
(ii) Chemical Equations
-
Dehydration of Ethanol (A → B):
-
Hydrogenation of Ethene (B → C):
(iii) Combustion of Compound C (Ethane)
Products: Carbon dioxide and water.
(iv) Industrial Application of Hydrogenation
- Hydrogenation of vegetable oils to form solid fats like ghee and margarine.
(v) Reaction of Compound A (Ethanol) with Sodium
Products: Sodium ethoxide and hydrogen gas.
(b) Soap, Detergent, and Hard Water
(i) Micelle Formation (Diagram)
- Soap molecules have a hydrophobic tail (non-polar) and hydrophilic head (polar).
- The tails attach to oil or grease, forming spherical structures called micelles that trap dirt and allow it to be washed away with water.
(ii) Foam Formation with Soap vs. Detergent
-
Test tube with more foam: Detergent (Test tube Y)
- Detergents work in both soft and hard water because they don’t form scum with calcium and magnesium ions.
-
Test tube with curdy solid: Soap (Test tube X)
- Soap reacts with hard water ions (Ca²⁺/Mg²⁺) to form an insoluble curdy precipitate (soap scum).
Question 35: Reproduction in Flowers 🌸
(a) Parts of a bisexual flower not involved in reproduction:
- Sepals (Calyx) – Protects the flower during bud stage.
- Petals (Corolla) – Attracts pollinators but doesn’t directly help in reproduction.
(b) Self-Pollination vs. Cross-Pollination
| Self-Pollination | Cross-Pollination |
|---|---|
| Transfer of pollen within the same flower or between flowers of the same plant. | Transfer of pollen between flowers of different plants. |
| Requires fewer resources. | Promotes genetic variation. |
Significance:
- Ensures reproduction even in isolated conditions (self-pollination).
- Increases genetic diversity (cross-pollination).
(c) Fate of Ovule and Ovary After Fertilization
- Ovule → Develops into a seed.
- Ovary → Develops into a fruit.
Question 36: Electric Iron & Heating Effect ⚡
(a) Calculate Current and Resistance
- Given:
- Maximum power (P₁) = 880 W
- Minimum power (P₂) = 330 W
- Voltage (V) = 220 V
Formula:
and
-
Current at Maximum Power:
-
Current at Minimum Power:
(b) Heating Effect of Electric Current:
- When current passes through a conductor, it generates heat due to the resistance of the conductor (Joule’s Law of Heating).
(c) Expression for Heat Produced:
Where:
-
= Current (A)
-
= Resistance (Ω)
-
= Time (s)
SECTION – E
Question 37: Metal Oxides and Reactivity 🔥
(a) Copper Heated in Air:
Product: Copper(II) oxide – a black solid.
(b) Amphoteric Oxides:
- Oxides that react with both acids and bases.
- Example: Aluminium oxide (Al₂O₃) reacts with HCl (acid) and NaOH (base).
(c) Complete the Equations:
-
-
(Sodium aluminate + water)
Question 37 (c): Sulfur Burning in Oxygen 🌫️
(i) Chemical Equation:
(ii) Gas Formed: Sulfur dioxide (SO₂)
(iii) Nature of Gas:
- Acidic in nature (forms sulfurous acid when dissolved in water).
(iv) Action on Dry Litmus Paper:
- No color change on dry litmus paper (needs moisture to form acid and turn red).
Question 38: Mendel’s Experiment 🌱
(a) Two Pairs of Contrasting Characteristics (Other than Seed Shape/Color):
- Flower color: Purple vs. White
- Plant height: Tall vs. Dwarf
(b) Dominant vs. Recessive Traits:
| Dominant Trait | Recessive Trait |
|---|---|
| Expressed in both homozygous (TT) and heterozygous (Tt) conditions. | Expressed only in homozygous condition (tt). |
| Masks the effect of recessive traits. | Masked in the presence of a dominant trait. |
Example:
- Tall (T) – Dominant
- Dwarf (t) – Recessive
(c) F₂ Generation Ratio for Shape and Color:
Phenotypic Ratio: 9:3:3:1
- 9: Round Yellow
- 3: Round Green
- 3: Wrinkled Yellow
- 1: Wrinkled Green
Interpretation:
- Confirms independent assortment of traits (Mendel’s Law of Independent Assortment).
OR (c) Violet × White Flower Cross:
Parent Generation (P):
- Pure violet (VV) × Pure white (vv)
F₁ Generation (VV × vv):
- All Vv (violet-flowered, dominant trait expressed).
F₂ Generation (Self-Pollination of Vv × Vv):
- Phenotypic Ratio: 3 Violet : 1 White
- Genotypic Ratio: 1 VV : 2 Vv : 1 vv
Question 39: Concave Mirror Applications and Calculations
(a) Applications of Concave Mirrors:
- Shaving/Makeup Mirrors: Magnifies the image for better visibility.
- Headlights of Vehicles: Reflects light in a strong, focused beam.
(b) Radius of Curvature Calculation:
Given: Focal Length (f) = 15 cm
The relationship between the radius of curvature (R) and the focal length (f):
Answer: 30 cm
(c) Ray Diagram – Object between Pole and Focus (Concave Mirror)
Characteristics of Image:
- Position: Behind the mirror
- Nature: Virtual, erect, and magnified
OR (c) Mirror Formula and Magnification Calculation
Given:
- Object Distance (u) = -100 cm
- Image Distance (v) = -100 cm (since the image is at the same location)
- Object Height (hₒ) = 10 cm
(i) Focal Length Calculation:
Mirror Formula:
Answer: Focal length = -50 cm (concave mirror has a negative focal length).
(ii) Magnification Calculation (M):
Interpretation:
- -1 Magnification: Image is same size as object, inverted, and real.